Introduction
The Alfacalcidol Production Process with Cost Analysis explores the intricate process of manufacturing alfacalcidol, a valuable vitamin D analogue used for managing conditions related to calcium and phosphorus metabolism, such as osteoporosis and renal osteodystrophy. The growing global demand for this essential compound in the healthcare industry necessitates a detailed understanding of its production process, cost drivers, raw material procurement, and market dynamics. This report offers an in-depth analysis, providing essential insights for businesses aiming to remain competitive in the alfacalcidol market.
Procurement Resource Assessment for Alfacalcidol Production Process
The Procurement Resource Assessment for alfacalcidol production focuses on sourcing high-quality raw materials and securing reliable suppliers for the key components necessary for manufacturing this vitamin D analogue. The procurement strategy is critical to ensuring consistent production and maintaining cost efficiency, especially given the sensitivity of alfacalcidol to external conditions like light and heat.
Request Free Sample – https://www.procurementresource.com/production-cost-report-store/alfacalcidol/request-sample
The primary raw materials required for alfacalcidol production include 7-dehydrocholesterol (a precursor to vitamin D3), various organic solvents, and reagents essential for the chemical transformation of the precursor into the active compound. Because of the precision required in this biochemical process, the quality of raw materials is paramount, and only pharmaceutical-grade sources can be used. This also means that procurement must comply with strict regulatory standards, such as Good Manufacturing Practices (GMP) and international pharmaceutical regulations.
In addition to sourcing the right materials, businesses must carefully evaluate global supply chains, as disruptions can lead to delays and increased costs. Establishing long-term relationships with trusted suppliers, diversifying sourcing strategies, and leveraging digital procurement platforms can help mitigate risks and ensure a steady flow of high-quality materials.
Understanding Alfacalcidol
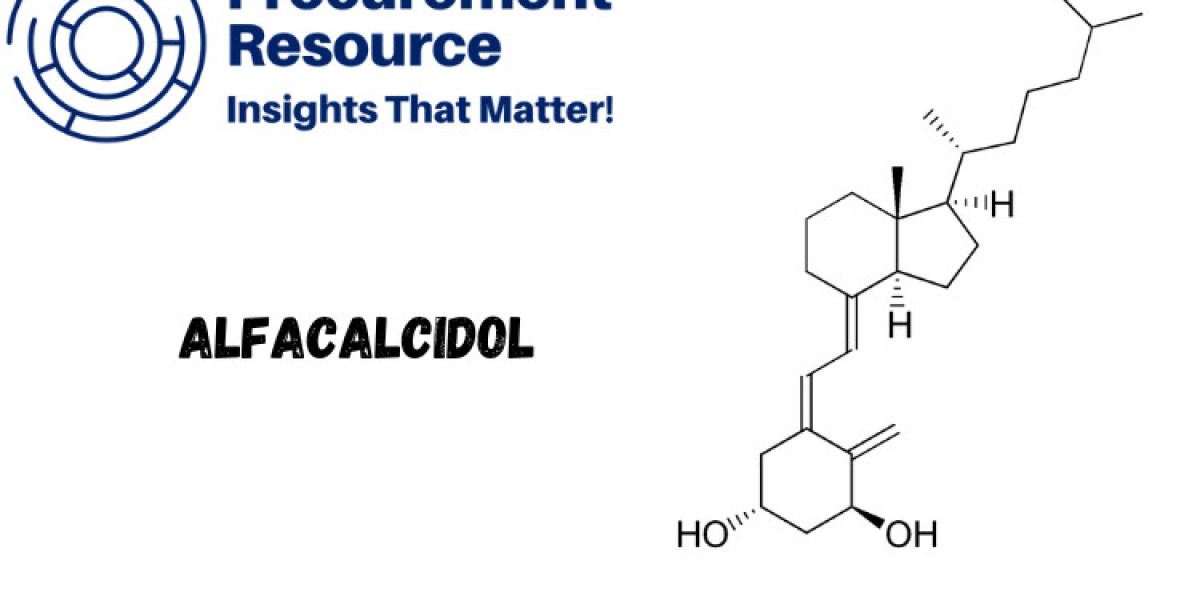
Alfacalcidol is a synthetic analogue of vitamin D, specifically designed to manage conditions related to calcium and phosphate metabolism in the body. It is a prodrug, meaning that it is converted into the active form, calcitriol (1,25-dihydroxyvitamin D3), by the liver. This makes alfacalcidol highly effective in treating conditions like osteoporosis, hypoparathyroidism, and chronic kidney disease (CKD), where calcium absorption and bone health are compromised.
Due to its rapid and controlled activation in the liver, alfacalcidol has gained significant attention in the medical field for its efficacy in managing bone-related disorders without the risk of hypercalcemia often associated with other forms of vitamin D. It is commonly prescribed in both oral and intravenous forms and is available globally under various brand names.
The increasing prevalence of osteoporosis, especially in aging populations, combined with the growing incidence of CKD, has expanded the demand for alfacalcidol in global pharmaceutical markets. Understanding its production process is critical for manufacturers aiming to meet this rising demand efficiently.
Market Drivers
Several key market drivers are influencing the production and demand for alfacalcidol across global markets. These drivers include:
Growing Incidence of Osteoporosis: As the global population ages, the prevalence of osteoporosis and related bone disorders is increasing significantly. Alfacalcidol’s role in promoting calcium absorption and improving bone mineral density has made it a preferred treatment option for elderly patients, driving demand in this segment.
Increased Prevalence of Chronic Kidney Disease (CKD): CKD impairs the kidneys’ ability to convert vitamin D into its active form, leading to calcium and phosphorus imbalances. Alfacalcidol bypasses this conversion process by being activated in the liver, making it a critical treatment for patients with CKD and renal osteodystrophy.
Rising Demand for Vitamin D Analogues: As awareness grows about the importance of vitamin D in maintaining bone health and preventing diseases, the demand for vitamin D analogues like alfacalcidol has risen sharply. This trend is particularly strong in regions with high incidences of vitamin D deficiency, such as parts of Asia and Europe.
Increasing Healthcare Expenditure: Expanding healthcare infrastructure, particularly in emerging markets, is boosting access to advanced treatments like alfacalcidol. Governments and healthcare organizations are investing in better management of chronic diseases, further driving demand for this essential medication.
New Drug Development and Research: The pharmaceutical industry continues to invest in the development of new formulations and delivery systems for alfacalcidol, such as extended-release capsules and injectable forms. Ongoing research into its potential benefits in treating other conditions, such as rheumatoid arthritis, is also contributing to market growth.
These market drivers underline the expanding role of alfacalcidol in global healthcare, presenting lucrative opportunities for manufacturers who can optimize their production processes.
Raw Materials Requirements
The production of alfacalcidol requires several specialized raw materials, with stringent purity and quality standards. Key raw materials include:
7-Dehydrocholesterol: This precursor compound is derived from cholesterol and undergoes a photochemical transformation to produce the vitamin D analogue. It is one of the most important raw materials in the production process, and its quality directly impacts the yield and efficacy of alfacalcidol.
Organic Solvents: Various solvents, such as ethanol and methanol, are used in the extraction and purification stages of the production process. These solvents must meet pharmaceutical-grade specifications to ensure that the final product is free from contaminants.
Reagents for Photochemical Reaction: Specialized reagents are required to facilitate the photochemical conversion of 7-dehydrocholesterol into alfacalcidol. These reagents must be sourced from reliable suppliers to ensure consistency in the reaction.
Antioxidants and Stabilizers: Given the sensitivity of alfacalcidol to light and heat, antioxidants and stabilizers are essential for maintaining the stability of the final product during storage and distribution.
High-Purity Water: High-purity water is needed for the washing, extraction, and crystallization steps in the production process. The water used must comply with pharmaceutical industry standards to prevent contamination and ensure product safety.
Securing reliable and high-quality sources for these raw materials is crucial for maintaining product consistency and meeting regulatory standards. Additionally, cost fluctuations in the supply of 7-dehydrocholesterol and solvents can significantly impact the overall production costs, making efficient procurement strategies essential for manufacturers.
Costs and Key Process Information
The Alfacalcidol Production Process involves several key stages, each of which contributes to the overall cost structure. The main production steps are as follows:
Photochemical Conversion: The process begins with the photochemical conversion of 7-dehydrocholesterol into alfacalcidol using UV light. This is a highly controlled process requiring specific wavelengths of light and precise timing to ensure optimal yields. The setup for this stage includes UV reactors and safety systems to manage light exposure.
Purification and Crystallization: After the photochemical conversion, the crude alfacalcidol must be purified. This involves solvent extraction, filtration, and crystallization to remove impurities and produce a high-purity product suitable for pharmaceutical use. Solvent recovery systems are often employed to reduce waste and control costs during this stage.
Stabilization and Formulation: Once purified, the alfacalcidol is stabilized using antioxidants to protect it from degradation due to light and heat. The stabilized product is then formulated into the desired dosage forms, such as capsules, tablets, or injectables. This step requires precise formulation techniques to ensure consistent dosing and product safety.
Packaging and Quality Control: The final product is packaged in UV-protected containers to prevent degradation during storage and distribution. Extensive quality control measures are employed to ensure that the product meets all regulatory requirements and is free from contaminants.
Cost Factors
Several key factors influence the overall cost of producing alfacalcidol, including:
Raw Material Costs: The price of 7-dehydrocholesterol, solvents, and reagents is a significant cost component in the production process. Global supply fluctuations, particularly in the availability of cholesterol derivatives, can impact raw material costs.
Energy Consumption: The photochemical conversion process requires a significant amount of energy, particularly for operating UV reactors and maintaining controlled temperatures. Energy costs, therefore, play a substantial role in determining overall production expenses.
Labor and Equipment Costs: Skilled labor is needed to operate the sophisticated equipment used in the production process. Additionally, the cost of maintaining UV reactors, crystallizers, and other specialized equipment must be factored into the overall production budget.
Regulatory Compliance: Ensuring that the production process meets stringent pharmaceutical industry regulations, including GMP standards, adds to the production costs. Regular audits, certifications, and quality control measures are necessary to comply with regulatory requirements in different markets.
Looking for an Exhaustive and Personalized Report?
For businesses looking to optimize their alfacalcidol production process or enter this expanding market, an exhaustive and personalized report can offer valuable insights. These reports provide:
Detailed Market Analysis: Offering a thorough breakdown of current market trends, growth forecasts, and demand drivers for alfacalcidol across different regions and sectors.
Cost Optimization Strategies: Including recommendations for reducing raw material costs, energy consumption, and labor expenses, while maintaining high-quality production standards.
Sustainability and Regulatory Compliance Guidance: Helping businesses navigate the complex regulatory landscape and adopt sustainable production practices that align with global industry trends.
By investing in a personalized report, companies can gain a competitive edge, improve their production processes, and position themselves for long-term success in the alfacalcidol market.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA